
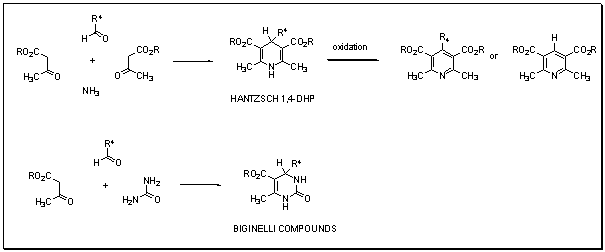
1,4-Dihydropyridines and Pyrimidines
Our work covers synthetic methodologies to access numerous 1,4-dihydropyridines, which are used as calcium antagonists, the corresponding pyridines, as well as structurally related pyrimidines (Biginelli compounds).Multi-component reactions are involved in those syntheses.
Nifedipine, a drug introduced in the USA in 1982, belongs to the class of the calcium channel blockers, also referred to as calcium antagonists. Clinical applications of this group of drugs include angina pectoris, hypertension, and supraventricular and ventricular arrythmias.
External Link: Article from Medicinenet over Nifedipine
The metabolism of Nifedipine and related 1,4-dihydropyridines involves an oxidation step that is catalyzed, in the liver, by cytochrome P-450. That reaction can be mimicked in cell-free systems and has been the subject of numerous publications.
- 1,3-Dicarbonyl Compound as Third Component (Hantzsch Pyridine Synthesis).
- Batch and Continuous Flow Preparation of Hantzsch 1,4-Dihydropyridines under Microwave Heating and Simultaneous Real-time Monitoring by Raman Spectroscopy. An Exploratory Study.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Preparation of New 3,4-Dihydropyrimidine-2(1H)-ones Bearing Bioisostere Group in N-3 Position.
- Sequential Synthesis of a New Analogue of Amlodipine Bearing a Short Amino Polyethyleneglycol Chain.
- A Three-component Condensation Protocol Based on Ionic Liquid Phase Bound Acetoacetate for the Synthesis of Biginelli 3,4-Dihydropyrimidine-2(1H)-ones.
- A New Approach to N-3 Functionalized 3,4-Dihydropyrimidine-2(1H)-ones With 1,2,4- Oxadiazole Group as Amide Isostere via Ionic Liquid Phase Technology.
- Liquid-phase Synthesis of Polyhydroquinoline Using Task-specific Ionic Liquid Technology.
- Ionic Liquid Phase Technology Supported the Three Component Synthesis of Hantzsch 1,4-Dihydropyridines and Biginelli 3,4-Dihydropyrimidin-2(1H)-ones under Microwave Dielectric Heating.
- New Pyridine-containing Non-cyclic and Macrocyclic Schiff Bases: Synthesis and Interferon-inducing Activity.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Three Component Preparation of 2-Thioxo Tetrahydropyrimidin-4(1H)-ones under Microwave Dielectric Heating.
- Syntheses and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview.
- Polymer-assisted Synthesis of Ethyl 2-Amino-4,6-diarylpyrimidine-5-carboxylates.
- Insoluble versus Soluble Polymer-assisted Synthesis. A First Approach for the Preparation of a Biginelli Compound.
- Hydrogen Transfer from Hantzsch 1,4-Dihydropyridines to Carbon-carbon Double Bonds under Microwave Irradiation.
- The Analysis of Structure-anticancer and Antiviral Activity relationships for Macrocyclic pyridinophanes and their Analogues on the Basis of 4D QSAR Models
- Anticancer and Antiviral Properties of Macrocyclic Pyridinophanes and their Derivatives.
- New Methodologies for the Preparation of Drugs and their Metabolites. Application to 1,4-dihydropyridines Structurally Related to Calcium Channel Modulators of the Nifedipine®-type.
- En Route to Macrocyclic Pyridinophanes as Anticancer Drugs. Synthetic Ways to the Building Blocks.
- Evidence for a Robinson-like Annulation during the Reaction Between a N-(1-chloroalkyl)pyridinium Chloride and a N-Substituted Enaminoester.
- Chemistry of N-(1-Haloalkyl)heteroarylium Salts.
- Microwave-mediated Derivatization of Poly(styrene-co-allyl alcohol), a Key Step for the Soluble Polymer-assisted Synthesis of Heterocycles.
- Synthesis and Aromatization of Dihydropyrimidines Structurally Related to Hantzsch Calcium Channel Modulators.
- Microwave-mediated Domino Reactions in Dry Medium. Preparation of Dihydropyridinones and Pyridinones Structurally related to Hantzsch Esters.
- Old Reagents, New Results : Aromatization of Hantzsch 1,4-Dihydropyridines with Manganese Dioxide and 2,3- Dichloro-5,6-dicyano-1,4- benzoquinone.
- Bentonite K10 Clay, an Efficient Catalyst for the Formation of Nitrogen Derivatives.
- Potassium Permanganate, a Versatile Reagent for the Aromatization of Hantzsch 1,4-Dihydropyridines.
- An Unusual Aromatization of 4-Antipyryl Hantzsch-type 1,4-Dihydropyridines.
- Rate-determining Effects in the Formation of Hantzsch 1,4-Dihydropyridines from N-(1-Haloalkyl)azinium Halides and Methyl 3-Amino-2-butenoate.
- Novel Syntheses of HeterocyÂcles with N-(1-Haloalkyl)azinium Halides. Part 5. Preparation of N-Substituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 2. Preparation of N-Unsubstituted 1,4-Dihydropyridines.
- A Novel Application of the Oxidizing Properties of Pyridinium Chloro-chromate: Aromatization of Hantzsch 1,4-DihydropyridiÂnes.
- Ultrasound-promoted Aromatization of Hantzsch 1,4-DihydropyÂridines by Clay-supported Cupric Nitrate.



