
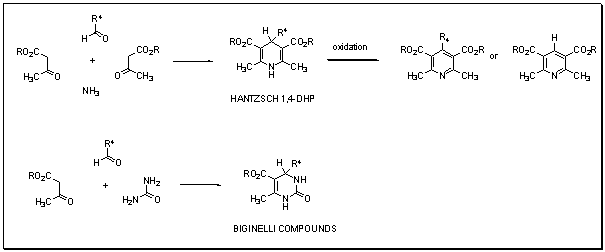
1,4-Dihydropyridines et Pyrimidines
Notre travail couvre des m├®thodologies de synth├©se pour acc├®der ├Ā de nombreuses 1,4-dihydropyridines, qui sont utilis├®es comme antagonistes du calcium, aux pyridines correspondantes, ainsi quŌĆÖaux pyrimidines analogues (composes de Biginelli).Des r├®actions multi-composants sont impliqu├®es dans ces synth├©ses.
La Nifedipine, un m├®dicament introduit aux USA en 1982, appartient ├Ā la classe des bloquants des canaux calciques, aussi appel├®s antagonistes du calcium. Les applications cliniques de ce groupe de medicaments couvent lŌĆÖangine de poitrine, lŌĆÖhypertension et les arythmies ventriculaire et supraventriculaire.
Link: Article de Medicinenet au sujet de la Nifedipine
Le m├®tabolisme de la Nifedipine et des 1,4-dihydropyridines analogues implique une ├®tape dŌĆÖoxydation qui est catalys├®e, dans le foie, par un cytochrome P-450. Cette r├®action peut-├¬tre reproduite en milieu abiotique et est le sujet de nombreuses publications.
- 1,3-Dicarbonyl Compound as Third Component (Hantzsch Pyridine Synthesis).
- Batch and Continuous Flow Preparation of Hantzsch 1,4-Dihydropyridines under Microwave Heating and Simultaneous Real-time Monitoring by Raman Spectroscopy. An Exploratory Study.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Preparation of New 3,4-Dihydropyrimidine-2(1H)-ones Bearing Bioisostere Group in N-3 Position.
- Sequential Synthesis of a New Analogue of Amlodipine Bearing a Short Amino Polyethyleneglycol Chain.
- A Three-component Condensation Protocol Based on Ionic Liquid Phase Bound Acetoacetate for the Synthesis of Biginelli 3,4-Dihydropyrimidine-2(1H)-ones.
- A New Approach to N-3 Functionalized 3,4-Dihydropyrimidine-2(1H)-ones With 1,2,4- Oxadiazole Group as Amide Isostere via Ionic Liquid Phase Technology.
- Liquid-phase Synthesis of Polyhydroquinoline Using Task-specific Ionic Liquid Technology.
- Ionic Liquid Phase Technology Supported the Three Component Synthesis of Hantzsch 1,4-Dihydropyridines and Biginelli 3,4-Dihydropyrimidin-2(1H)-ones under Microwave Dielectric Heating.
- New Pyridine-containing Non-cyclic and Macrocyclic Schiff Bases: Synthesis and Interferon-inducing Activity.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Three Component Preparation of 2-Thioxo Tetrahydropyrimidin-4(1H)-ones under Microwave Dielectric Heating.
- Syntheses and Aromatization of Hantzsch 1,4-Dihydropyridines under Microwave Irradiation. An Overview.
- Polymer-assisted Synthesis of Ethyl 2-Amino-4,6-diarylpyrimidine-5-carboxylates.
- Insoluble versus Soluble Polymer-assisted Synthesis. A First Approach for the Preparation of a Biginelli Compound.
- Hydrogen Transfer from Hantzsch 1,4-Dihydropyridines to Carbon-carbon Double Bonds under Microwave Irradiation.
- The Analysis of Structure-anticancer and Antiviral Activity relationships for Macrocyclic pyridinophanes and their Analogues on the Basis of 4D QSAR Models
- Anticancer and Antiviral Properties of Macrocyclic Pyridinophanes and their Derivatives.
- New Methodologies for the Preparation of Drugs and their Metabolites. Application to 1,4-dihydropyridines Structurally Related to Calcium Channel Modulators of the Nifedipine®-type.
- En Route to Macrocyclic Pyridinophanes as Anticancer Drugs. Synthetic Ways to the Building Blocks.
- Evidence for a Robinson-like Annulation during the Reaction Between a N-(1-chloroalkyl)pyridinium Chloride and a N-Substituted Enaminoester.
- Chemistry of N-(1-Haloalkyl)heteroarylium Salts.
- Microwave-mediated Derivatization of Poly(styrene-co-allyl alcohol), a Key Step for the Soluble Polymer-assisted Synthesis of Heterocycles.
- Synthesis and Aromatization of Dihydropyrimidines Structurally Related to Hantzsch Calcium Channel Modulators.
- Microwave-mediated Domino Reactions in Dry Medium. Preparation of Dihydropyridinones and Pyridinones Structurally related to Hantzsch Esters.
- Old Reagents, New Results : Aromatization of Hantzsch 1,4-Dihydropyridines with Manganese Dioxide and 2,3- Dichloro-5,6-dicyano-1,4- benzoquinone.
- Bentonite K10 Clay, an Efficient Catalyst for the Formation of Nitrogen Derivatives.
- Potassium Permanganate, a Versatile Reagent for the Aromatization of Hantzsch 1,4-Dihydropyridines.
- An Unusual Aromatization of 4-Antipyryl Hantzsch-type 1,4-Dihydropyridines.
- Rate-determining Effects in the Formation of Hantzsch 1,4-Dihydropyridines from N-(1-Haloalkyl)azinium Halides and Methyl 3-Amino-2-butenoate.
- Novel Syntheses of Heterocy┬Łcles with N-(1-Haloalkyl)azinium Halides. Part 5. Preparation of N-Substituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 2. Preparation of N-Unsubstituted 1,4-Dihydropyridines.
- A Novel Application of the Oxidizing Properties of Pyridinium Chloro-chromate: Aromatization of Hantzsch 1,4-Dihydropyridi┬Łnes.
- Ultrasound-promoted Aromatization of Hantzsch 1,4-Dihydropy┬Łridines by Clay-supported Cupric Nitrate.



