
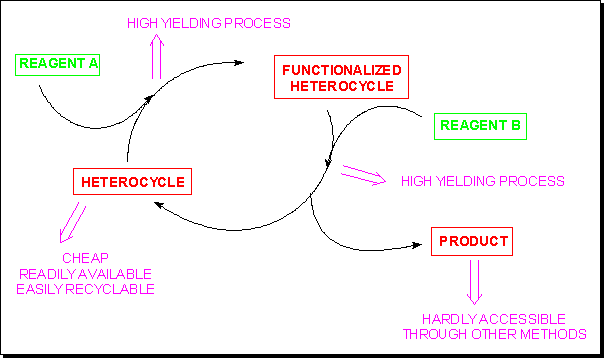
Heterocycle-assisted syntheses
Just like a halogen atom or a diazonium group, several neutral or charged heterocyclic moieties can act as efficient leaving groups and therefore be considered as useful synthetic auxiliaries, provided:- the starting heterocycle is cheap or readily available;
- the heterocyclic derivative can be prepared in high yield;
- the heterocyclic moiety can be readily displaced;
- the target molecule can be isolated in high yield and should be hardly obtained by another synthetic pathway;
- the heterocyclic moiety can be readily recyclable.
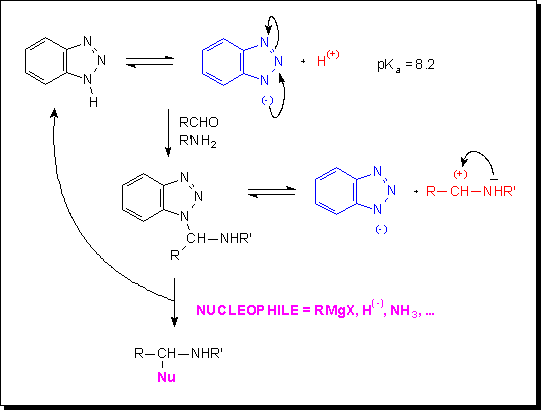
Numerous benzotriazole derivatives as well as pyridinium salts fulfill those requirements.
Benzotriazole is a weak acid (pKa ~ 8, like penta-2,4-dione); it readily reacts, i.a., with an aldehyde and an amine in a three-component synthesis to yield an aminal for which an equilibrium with corresponding charged species can be considered. Further reaction with a nucleophile is accompanied by the displacement of the benzotriazole anion and affords a highly substituted amine. As demonstrated by A.R. Katritzky et al., that is only one example of the numerous synthetic possibilities offered by the benzotriazole chemistry.
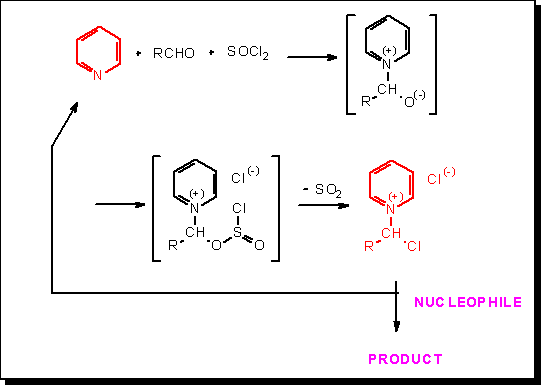
In 1987, E. Anders and J. Tropsch (Bull. Soc. Chim. Belges, 96, 719 – 720, 1987) described the first synthesis of N-(1-chloroalkyl)pyridinium chlorides from pyridine, aldehydes, and thionyl chloride. We have subsequently proven that such salts represent useful activated equivalents of the corresponding starting aldehydes. Let us emphasize, among the various examples we studied, that those pyridinium derivatives can be involved in the preparation of Hantzsch esters (N-unsubstituted as well as N-substituted) and in the direct synthesis of benzimidazoles, imidazopyridines, as well as perimidines, without the need for an external oxidant.
- 1,3-Dicarbonyl Compound as Third Component (Hantzsch Pyridine Synthesis).
- Batch and Continuous Flow Preparation of Hantzsch 1,4-Dihydropyridines under Microwave Heating and Simultaneous Real-time Monitoring by Raman Spectroscopy. An Exploratory Study.
- Design and synthsesis of N1,N5-bis[4-(5-alkyl-1,2,4-oxadiazole-3-yl)phenyl]glutaramides as potential antifungal prodrugs.
- Fragment-based Design of Symmetrical Bis-benzimidazoles as Selective Inhibitors of the Trimethoprim-Resistant, Type IIR67.
- 1,4-Diarylpiperazine and Analogs as Anti-tubercular Agents: Synthesis and Biological Evaluation.
- Bis(oxyphenylene)benzimidazoles: a Novel Class of Anti-Plasmodium falciparum Agents.
- Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles Using Ionic Liquid-phase Organic Synthesis (IoLiPOS) Methodology.
- Synthesis and Application of Ionic Liquid Phase-Supported ß-Aminocrotonate for Access of Asymmetric 1,4-Dihydropyridines.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Preparation of New 3,4-Dihydropyrimidine-2(1H)-ones Bearing Bioisostere Group in N-3 Position.
- From a Three-component Synthesis to Multistep Cascade Reactions. Twenty Years in the Chemistry of N-(1-Haloalkyl)azinium Halides.
- Sequential Synthesis of a New Analogue of Amlodipine Bearing a Short Amino Polyethyleneglycol Chain.
- A New Approach to N-3 Functionalized 3,4-Dihydropyrimidine-2(1H)-ones With 1,2,4-Oxadiazole Group as Amide Isostere via Ionic Liquid Phase Technology.
- A Three-component Condensation Protocol Based on Ionic Liquid Phase Bound Acetoacetate for the Synthesis of Biginelli 3,4-Dihydropyrimidine-2(1H)-ones.
- Liquid-phase Synthesis of Polyhydroquinoline Using Task-specific Ionic Liquid Technology.
- Ionic Liquid Phase Technology Supported the Three Component Synthesis of Hantzsch 1,4-Dihydropyridines and Biginelli 3,4-Dihydropyrimidin-2(1H)-ones under Microwave Dielectric Heating.
- Novel Isothiocyanato Ester Appended to Task Specific Ionic Liquid as New Tools for Ionic Liquid Phase Organic Synthesis (Iolipos).
- New Methodologies for the Preparation of Drugs and their Metabolites. Application to 1,4-Dihydropyridines structurally related to Calcium Channel Modulators of the Nifedipineâ„¢-type.
- Chemistry of N-(1-Haloalkyl)Heteroarylium Salts.
- En route to Macrocyclic Pyridinophanes as Anticancer Drugs. Synthetic Ways to the Building Blocks
- Evidence for a Robinson-like Annelation during the Reaction between N-(1-Chloroalkyl)pyridinium Chlorides and N-substituted Enaminoesters.
- 1-(Arylchloromethyl)pyridinium Chlorides. Investigation by X-ray Single Crystal Diffraction and Semiempirical (PM3, AM1, MNDO) Calculations.
- Synthesis of Thiazolidine-4-carboxylic Acids and N-(1-Sulfonatoalkyl)pyridinium Betaines from N-(1-Chloroalkyl)pyridinium Chlorides.
- Highly Selective Alkylation of 5-Amino-1-methyl-1H-1,2,4-triazole with N-(1-Chloroalkyl)pyridinium Chlorides under Formation of Novel Geminal Bis-Heteroarylium Salts: a Combined Experimental / MO-theoretical Study.
- An Unusual Aromatization of 4-Antipyryl Hantzsch-type 1,4-Dihydropyridines.
- An Expedient Route to 1H-Benzimidazoles and 1H-Imdazopyridines.
- A New and Convenient Method for the Preparation of 2-Substituted Quinazolines.
- Rate-determining Effects in the Formation of Hantzsch 1,4-Dihydropyridines from N-(1-Haloalkyl)azinium Halides and Methyl 3-Amino-2-butenoate.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 6. Preparation of 2,3,4,5,10,11-Hexahydro-1H -Dibenzo[b,e][1,4]diazepin-1-ones.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 5. Preparation of N-Substituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 4. An Unexpected One-pot Preparation of 1H-Perimidines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 3. Preparation of 2-Aryl-1-arylimino-2,3-dihydro-1H-isoindoles.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 2. Preparation of N-Unsubstituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 1. Preparation of Imidazolidines.
- Preparation of 2-phenyl-1H-Benzimidazoles.
- Rate-determining Effects in the Formation of N-(1-haloalkyl)heteroarylium Halides.
- Monoacyl Aminals and their Peptide Derivatives.
- N-[(2-naphtyloxy)methyl]benzazoles : Synthesis and Investigations by X-ray Analysis and by Semiempirical Calculations
- The Chemistry of N-substituted Benzotriazoles. Part 23. Synthesis of tertiary -Aminoesters.
- Benzotriazole-assisted Synthesis of Monoacyl -Aminoglycines.
- N-(1-Haloalkyl)pyridinium Salts : Preparation and Use for New Syntheses of Other N-(1-substituted-alkyl)pyridinium Salts, N,N'-(1-alkylidene)bisamines, and N,N'-(1-alkylidene)-bisbenzazoles.
- The Chemistry of N-substituted Benzotriazoles. Part 20. Mono-N-t-Butylation of Aromatic and Heteroaromatic Amines.
- Synthesis of Imines from N-(1-Halogenoalkyl)heteroarylium Salts.
- Solvent-free Heterocyclic Synthesis M.A.P. MARTINS, C.P. FRIZZO, D.N. MOREIRA, L. BURIOL, P. MACHADO. Chem. Rev., 109, 4140 (2009).
- Benzotriazole Mediated Amino-, Amido-, Alkoxy-, and Alkylthio-alkylation. A.R. KATRITZKY, K. MANIU, S.K. SINGH, N.K. MEHER. Tetrahedron, 61, 2555 (2005).
- The Continuing Magic of Benzotriazole: an Overview of some Recent Advances in Synthetic Methodology. A.R. KATRITZKY. J. Heterocycl. Chem., 36, 1501 (1999).
- Highlights from Fifty Years of Heterocyclic Chemistry. A.R. KATRITZKY. J. Heterocycl. Chem., 31, 569 (1994).
- N-Substituted Benzotriazoles : Properties, Reactivities and Synthetic Utility. A.R. KATRITZKY. Bull. Soc. Chim. Belg., 101, 409 (1992).
- Benzotriazole : a Novel Synthetic Auxiliary. A.R. KATRITZKY, S. RACHWAL, G.J. HITCHINGS. Tetrahedron, 47, 2683 (1991).



