
Les synthèses assistées par un heterocycle
Tout comme un atome d’halogène ou un groupe diazonium, certaines entités hétérocycliques neutres ou chargées peuvent jouer le rôle de groupe partant et donc être considérées comme de précieux auxiliaires de synthèse, pour autant que :- L’hétérocycle de départ soit bon marché et facilement disponible;
- Le dérivé hétérocyclique puisse être préparé avec un excellent rendement;
- L’auxiliaire hétérocyclique puisse être facilement déplacé;
- La molécule cible puisse être isolée avec un excellent rendement et soit difficilement accessible par une autre méthode;
- L’auxiliaire hétérocyclique puisse être facilement recyclé.
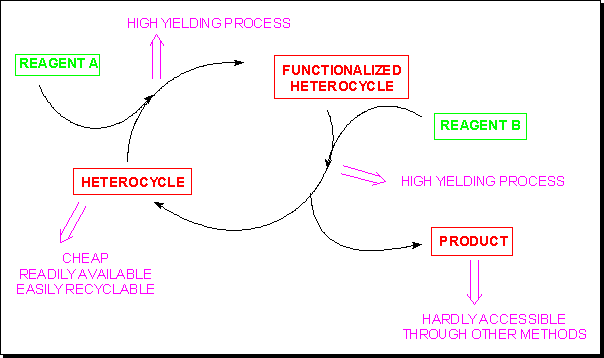
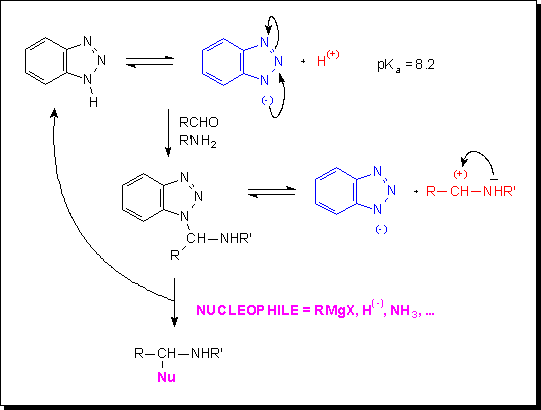
De nombreux dérivés du benzotriazole et des sels de pyridinium satisfont ces conditions.
Le benzotriazole est un acide faible (pKa ~ 8, comme la penta-2,4-dione); il réagit facilement, entre autres, avec un aldéhyde et une amine dans une réaction à trois composants pour fournir un aminal pour lequel un équilibre avec les formes chargées correspondantes peut être considéré. Une réaction subséquente avec un nucléophile est accompagnée du déplacement de l’anion benzotriazole et conduit, de la sorte, à une amine fortement substituée. Comme démontré par A.R. Katritzky et al., ceci est seulement l’un des nombreux exemples des possibilités offertes par la chimie du benzotriazole.
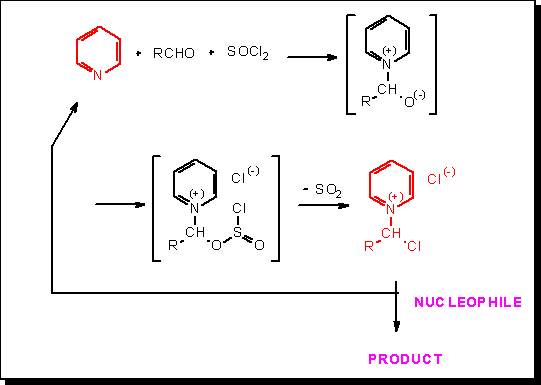
En 1987, E. Anders and J. Tropsch (Bull. Soc. Chim. Belges, 96, 719 – 720, 1987) ont décrit la première synthèse de chlorures de N-(1-chloroalkyl)pyridinium chlorides à partir de pyridine, d’aldéhydes et de chlorure de thionyle. Nous avons ensuite prouvé que ces sels représentent d’intéressantes formes activées des aldéhydes précurseurs. Soulignons, parmi les multiples exemples que nous avons étudiés, la préparation d’esters de Hantzsch (1,4-dihydropyridines, N-substituées ou non) et la synthèse directe de benzimidazoles, imidazopyridines et périmidines, sans la nécessité d’utiliser un agent oxydant extérieur.
- 1,3-Dicarbonyl Compound as Third Component (Hantzsch Pyridine Synthesis).
- Batch and Continuous Flow Preparation of Hantzsch 1,4-Dihydropyridines under Microwave Heating and Simultaneous Real-time Monitoring by Raman Spectroscopy. An Exploratory Study.
- Design and synthsesis of N1,N5-bis[4-(5-alkyl-1,2,4-oxadiazole-3-yl)phenyl]glutaramides as potential antifungal prodrugs.
- Fragment-based Design of Symmetrical Bis-benzimidazoles as Selective Inhibitors of the Trimethoprim-Resistant, Type IIR67.
- 1,4-Diarylpiperazine and Analogs as Anti-tubercular Agents: Synthesis and Biological Evaluation.
- Bis(oxyphenylene)benzimidazoles: a Novel Class of Anti-Plasmodium falciparum Agents.
- Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles Using Ionic Liquid-phase Organic Synthesis (IoLiPOS) Methodology.
- Synthesis and Application of Ionic Liquid Phase-Supported ß-Aminocrotonate for Access OF ASYMMETRIC 1,4-DIHYDROPYRIDINES.
- Ionic Liquid Phase Organic Synthesis (IoLiPOS) Methodology Applied to the Preparation of New 3,4-Dihydropyrimidine-2(1H)-ones Bearing Bioisostere Group in N-3 Position.
- From a Three-component Synthesis to Multistep Cascade Reactions. Twenty Years in the Chemistry of N-(1-Haloalkyl)azinium Halides.
- Sequential Synthesis of a New Analogue of Amlodipine Bearing a Short Amino Polyethyleneglycol Chain.
- A New Approach to N-3 Functionalized 3,4-Dihydropyrimidine-2(1H)-ones With 1,2,4-Oxadiazole Group as Amide Isostere via Ionic Liquid Phase Technology.
- A Three-component Condensation Protocol Based on Ionic Liquid Phase Bound Acetoacetate for the Synthesis of Biginelli 3,4-Dihydropyrimidine-2(1H)-ones.
- Liquid-phase Synthesis of Polyhydroquinoline Using Task-specific Ionic Liquid Technology.
- Ionic Liquid Phase Technology Supported the Three Component Synthesis of Hantzsch 1,4-Dihydropyridines and Biginelli 3,4-Dihydropyrimidin-2(1H)-ones under Microwave Dielectric Heating.
- Novel Isothiocyanato Ester Appended to Task Specific Ionic Liquid as New Tools for Ionic Liquid Phase Organic Synthesis (Iolipos).
- New Methodologies for the Preparation of Drugs and their Metabolites. Application to 1,4-Dihydropyridines structurally related to Calcium Channel Modulators of the Nifedipine™-type.
- Chemistry of N-(1-Haloalkyl)Heteroarylium Salts.
- En route to Macrocyclic Pyridinophanes as Anticancer Drugs. Synthetic Ways to the Building Blocks
- Evidence for a Robinson-like Annelation during the Reaction between N-(1-Chloroalkyl)pyridinium Chlorides and N-substituted Enaminoesters.
- 1-(Arylchloromethyl)pyridinium Chlorides. Investigation by X-ray Single Crystal Diffraction and Semiempirical (PM3, AM1, MNDO) Calculations.
- Synthesis of Thiazolidine-4-carboxylic Acids and N-(1-Sulfonatoalkyl)pyridinium Betaines from N-(1-Chloroalkyl)pyridinium Chlorides.
- Highly Selective Alkylation of 5-Amino-1-methyl-1H-1,2,4-triazole with N-(1-Chloroalkyl)pyridinium Chlorides under Formation of Novel Geminal Bis-Heteroarylium Salts: a Combined Experimental / MO-theoretical Study.
- An Unusual Aromatization of 4-Antipyryl Hantzsch-type 1,4-Dihydropyridines.
- An Expedient Route to 1H-Benzimidazoles and 1H-Imdazopyridines.
- A New and Convenient Method for the Preparation of 2-Substituted Quinazolines.
- Rate-determining Effects in the Formation of Hantzsch 1,4-Dihydropyridines from N-(1-Haloalkyl)azinium Halides and Methyl 3-Amino-2-butenoate.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 6. Preparation of 2,3,4,5,10,11-Hexahydro-1H -Dibenzo[b,e][1,4]diazepin-1-ones.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 5. Preparation of N-Substituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 4. An Unexpected One-pot Preparation of 1H-Perimidines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 3. Preparation of 2-Aryl-1-arylimino-2,3-dihydro-1H-isoindoles.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 2. Preparation of N-Unsubstituted 1,4-Dihydropyridines.
- Novel Syntheses of Heterocycles with N-(1-Haloalkyl)azinium Halides. Part 1. Preparation of Imidazolidines.
- Preparation of 2-phenyl-1H-Benzimidazoles.
- Rate-determining Effects in the Formation of N-(1-haloalkyl)heteroarylium Halides.
- Monoacyl Aminals and their Peptide Derivatives.
- N-[(2-naphtyloxy)methyl]benzazoles : Synthesis and Investigations by X-ray Analysis and by Semiempirical Calculations
- The Chemistry of N-substituted Benzotriazoles. Part 23. Synthesis of tertiary -Aminoesters.
- Benzotriazole-assisted Synthesis of Monoacyl -Aminoglycines.
- N-(1-Haloalkyl)pyridinium Salts : Preparation and Use for New Syntheses of Other N-(1-substituted-alkyl)pyridinium Salts, N,N'-(1-alkylidene)bisamines, and N,N'-(1-alkylidene)-bisbenzazoles.
- The Chemistry of N-substituted Benzotriazoles. Part 20. Mono-N-t-Butylation of Aromatic and Heteroaromatic Amines.
- Synthesis of Imines from N-(1-Halogenoalkyl)heteroarylium Salts.
- Solvent-free Heterocyclic Synthesis M.A.P. MARTINS, C.P. FRIZZO, D.N. MOREIRA, L. BURIOL, P. MACHADO. Chem. Rev., 109, 4140 (2009).
- Benzotriazole Mediated Amino-, Amido-, Alkoxy-, and Alkylthio-alkylation. A.R. KATRITZKY, K. MANIU, S.K. SINGH, N.K. MEHER. Tetrahedron, 61, 2555 (2005).
- The Continuing Magic of Benzotriazole: an Overview of some Recent Advances in Synthetic Methodology. A.R. KATRITZKY. J. Heterocycl. Chem., 36, 1501 (1999).
- Highlights from Fifty Years of Heterocyclic Chemistry. A.R. KATRITZKY. J. Heterocycl. Chem., 31, 569 (1994).
- N-Substituted Benzotriazoles : Properties, Reactivities and Synthetic Utility. A.R. KATRITZKY. Bull. Soc. Chim. Belg., 101, 409 (1992).
- Benzotriazole : a Novel Synthetic Auxiliary. A.R. KATRITZKY, S. RACHWAL, G.J. HITCHINGS. Tetrahedron, 47, 2683 (1991).



