


Computer controlled automated production (in collaboration with Ipratech)
![]()
Development of an automated capacitance-based, software-driven compact system allowing mammalian cells to be grown in sonoperfused submerged cultures under batch- (constant volume) or fedbatch (constant concentration) conditions with continuous monitoring of pH and dissolved oxygen at cell concentrations between 2- and 20 millions cells/ml. This system, together with its multiplexing capability (up to 4 bioreactors) and remote control via internet makes the following applications possible:
- Use in confined cGMP environment.
- Manufacture of constant quality cells at low, medium or high cell concentration.
- Rapid bioprocess optimization (specific growth rate, growth medium chemical composition, rate of perfusion, maximum obtained cell concentration etc…)
- Reduced human intervention which is limited to connecting/disconnecting feed (fresh growth medium) and waste (spent medium) vessels when required.
- Automated scale-up resulting in increasing the speed to market while decreasing costs.
___________________________________
Measurement and control of viable cell density in a mammalian cell bioprocessing facility: Validation of the software
D. Sergeant, Awardee of a Walloon Region "Bourse de préactivité", Mons/Belgium; M. Moser, CILBIOTECH sa, Mons/Belgium; J.P. Carvell, Aber Instruments Ltd, Aberystwyth/United Kingdom
Abstract
Because of the increased demand for cells growing in suspension (about 60-70% of all recombinant protein pharmaceuticals are produced in mammalian cells, most of which grow in continuously stirred tank reactor), ways are being sought to automate their production.
Many cell culture processes are based on perfusion or fed-batch bioreactor systems. Control of the feed or addition rates to maintain steady-state conditions in these bioreactors can be especially challenging due to high and fluctuating cell concentrations that can rapidly change environmental conditions. Simple control of perfusion bioreactors, based on infrequent daily sampling and estimation of the live cell concentration, will lead to large process deviations. Tight control of the perfusion or concentrate addition rate using real time, online cell concentration measurement allows the bioreactor to be operated under the optimum conditions for maximum recombinant protein production.
In this poster we report how a robust automatic perfusion rate control system based on on-line radio-frequency impedance probe has been used in perfused cytostat. The system operated in a completely closed loop i.e. no samples needed to be taken to obtain process information. In the control algorithm, a cell specific perfusion rate was specified and the cell density signal was converted into a perfusion flow rate. Example are shown of the actual time-dependent capacitance traces of a perfused Hela cell cultures evolving from batch (preset volume, increasing concentration) to fedbatch (increasing volume, preset cell concentration) with a preset cell concentration in the bioreactor.
Figure 1 presents the results of a 20-day culture of Hela cells inoculated at 2,5 x 106 cells/ml and ran automatically in batch mode until the pre-set concentration of 25x106 cells/ml is reached. As shown, the cell concentration then becomes automatically regulated and remains constant. The fluctuations in the volume of the culture represents daily sampling. Grown under such sonoperfused fedbatch ("cytostat") conditions, the only manipulations required consisted in changing regularly the feed-and waste bottles. Cell viability as measured by the trypan blue exclusion test averages 96%.
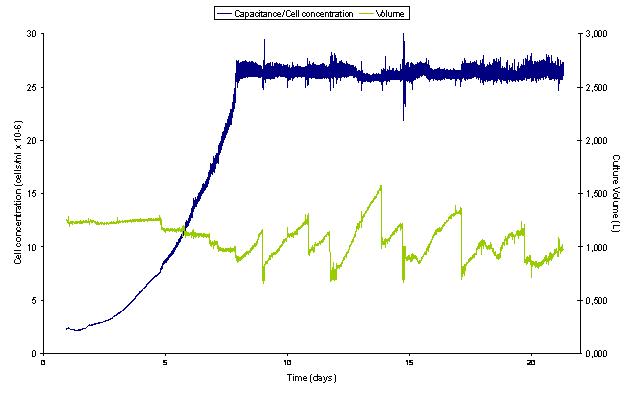
Figure 1: Automated cultivation of Hela cells in sonoperfused batch and fedbatch ("cytostat") modes: Hela cells have been grown in an Applikon 3L nominal capacity water-jacketed glass bioreactor equipped with dissolved oxygen and pH electrodes, the ADI 1030 Biocontroller, the BioSep ADI 1015 acoustic control unit and the 50L SonoSep acoustic cell filtration chamber. An Aber 340mm stainless steel capacitance probe is linked to the model 520 Viable Cell Monitor. This ensemble is operated as described in the literature (1) with some adaptations.
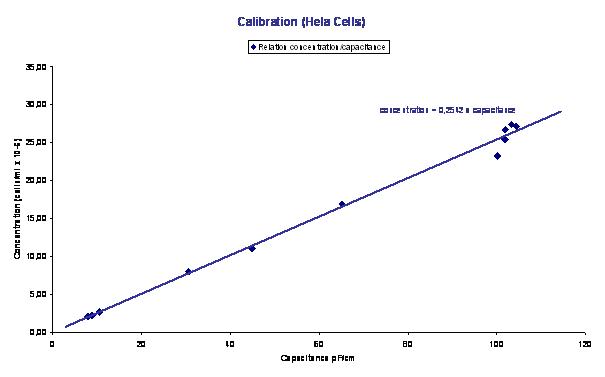
Figure 2 : Concentration versus capacitance plot obtained during the experiment depicted in Figure 1
The computer-controlled growth of mammalian cells in sonoperfused cytostat mode presents the following characteristics:
- Automated production of large numbers of constant quality cells
- Reduction of the culture volumes to be handled
- Reduction of human interventions
- Reduced footprint
The system lends itself ideally to be placed in a cGMP environment or confined in a restriction access barrier system.
Future developments consist in devising similar softwares specifically dedicated to the propagation of (genetically engineered) cell lines secreting therapeutic/prophylactic monoclonal antibodies, coagulation Factor VIII,…
References:
(1) Optimization of an Acoustic Cell Filter with a Novel Air-Backflush System
Biotechnol. Prog. 2003, 19, 30-36
Volker M. Gorenflo, Sumitra Angepat, Bruce D. Bowen and James M. Piret